The Benefits of Plant-Based Nutrition: Treatment and Prevention of Autoimmune Disease
Unlike traditional pharmaceutical treatments that can be costly and produce side effects, treatments involving plant-based nutrition are cost-effective, safe, and have strong potential to improve multiple health outcomes including autoimmune diseases.

Abstract
Healthy immune function is necessary to fight against viruses and diseases, yet when the immune system is poorly regulated, healthy cells can become the target of the immune system’s robust defenses. Such is the case with autoimmune diseases, which encompass type 1 diabetes, inflammatory bowel diseases, rheumatoid arthritis, and nervous system diseases like multiple sclerosis, among many other disorders. Autoimmune conditions are becoming increasingly common in the United States and are currently among the most common disease category affecting millions of Americans each year. One potential explanation for the increase is the rise in the obesity state, which can interfere with the body’s ability to regulate immune responses such as inflammation. Diet has strong associations with obesity, inflammation, and the immune system, and there is now ample evidence indicating that diets high in plant foods (e.g., fruits, vegetables, whole grains, legumes, nuts, and seeds) have beneficial effects on weight loss, markers of inflammation, and symptoms associated with autoimmune dysfunction. Unlike traditional pharmaceutical treatments that can be costly and produce unpleasant side effects, treatments involving dietary changes are cost-effective, safe, and have strong potential to improve multiple health outcomes beyond the targeted autoimmune diseases. With these benefits in mind, clinicians can consider plant-based dietary patterns as a component of the current treatment regimens for patients with autoimmune diseases.
Key Points for Practitioners
- Whole food, plant-based (WFPB) diets have utility both in decreasing risk for autoimmune conditions and, in some cases, improving symptoms among those currently diagnosed.86, 118, 119, 126
- Two major mechanisms by which the diet may play a role is through decreasing inflammation and by affecting the gut microbiome.118, 127–129
- WFPB diets have no adverse side effects and are associated with other health benefits130 such as cardiometabolic health,131–133 healthy weight,86, 134 and longevity.135
- Some evidence suggests that either or both gluten-free84, 86, 136, 137 or raw food versions of plant-based diets120, 138 may offer additional support.
- Existing evidence on plant-based diets and autoimmune conditions can be communicated to patients to support their ability to make informed decisions about their diet and course of treatment.
- If patients express interest in trying a WFPB diet of some kind, it is helpful to share ACLM patient-facing resources and tools with them.139–141
Autoimmune Disease in Context
Autoimmune disease, or autoimmune inflammatory (AI) disease, refers to some 80 to 100 or more related diseases1,2 that occur as a result of an overreactive immune system. Here, the body attacks healthy cells, tissues, or organs that it mistakes for foreign bodies, which results in the production of antibodies and creates a chronic inflammatory state.3 With so many potentially autoimmune related diseases, prevalence and incidence rates are difficult to calculate; however, the National Institutes of Health (NIH) estimates that 23.5 million Americans are currently affected by autoimmune diseases, while the American Autoimmune Related Diseases Association (AARDA) estimates a much higher 50 million.2 Globally, it is thought that roughly 4% of the world’s population is affected by at least one autoimmune disease,4 and the prevalence of these conditions continues to rise.5, 6
Different autoimmune diseases affect different organ systems. For instance, in people with Type 1 diabetes, the endocrine system is attacked, which inhibits hormonal production of insulin. In Crohn’s disease and other inflammatory bowel diseases, the digestive system is targeted. It is the musculoskeletal system under attack in rheumatoid arthritis, and autoimmune conditions can also affect the central nervous system (multiple sclerosis), as well as the skin and connective tissues (e.g., psoriasis).7, 8
Autoimmune diseases also affect certain populations disproportionately. In the United States, 78% of autoimmune cases occurred in women, according to a 2004 report from the Centers for Disease Control and Prevention (CDC).8 Women of African, Hispanic, Asian, and Native American descent have been more likely to develop lupus, for example, than Caucasian women, and African American women have also been shown to develop this disease at a younger age than Caucasians.7, 9
Autoimmune diseases are known to have a genetic basis s tend to cluster in families, but individual family members may experience different autoimmune diseases.7, 9 While genetics are largely implicated in the development of many autoimmune diseases, T regulatory cells seem to play a central role in autoimmune dysfunction related to pro-inflammatory cytokine secretion,10, 11 and circumstantial evidence links certain autoimmune dysfunctions to preceding infections.12–14
Diet and Autoimmune Diseases
Though it is generally agreed that genetic factors predispose individuals to the development of autoimmune disorders,15, 16 research (including twin studies) reveals that environmental factors and diet play a predominant role in the manifestation of these diseases.17
There is a higher prevalence of autoimmune diseases in Western societies where lifestyle may contribute through modern cleanliness, and sterilization practices reducing exposure to beneficial pathogens.13, 18, 19 Western dietary practices are also implicated, characterized by high intakes of energy, cholesterol, protein, saturated fat, added sugar, and salt with low intakes of fiber and antioxidants. Such a diet is associated with increased risk of autoimmune diseases through directly increasing inflammation20, 21 and indirectly increasing insulin resistance and obesity.22, 24
Non-Western countries that have experienced relatively lower rates of autoimmune disease like Japan and India are now seeing increased incidence, possibly associated with changing environmental and lifestyle factors25 such as diet.26, 27, 28 Though there is limited evidence that autoimmune diseases are directly linked to diet, this may be due to the challenge of detecting causal relationships in otherwise heterogeneous populations.10 Studies on individual nutrients have often born conflicting results.10, 29 With these challenges in mind, the remainder of this chapter will focus on existing evidence relating dietary factors to the conditions listed in Table 1, presently classified as an autoimmune disease.
A Note on Parkinson’s Disease
The National Parkinson’s Foundation clarifies that Parkinson’s disease (PD) is not categorized as an autoimmune disease at the time of this writing, as the cause is still unclear. Therefore, it is not included in this table and the summary of key studies to follow, though PD may be included in some research mentioned here.
The etiology of PD appears to be multifactorial, with potentially genetic as well as environmental factors at play. A recent study (2020) adds increasing evidence that PD is at least partly an autoimmune disease as it involves recognition by T cells of specific epitopes derived from the PD-associated protein α-synuclein.92, 93 The researchers report that signs of autoimmunity can appear in PD patients years before their official diagnosis.
Regarding interventions for PD patients, researchers have found improvements in the Unified Parkinson’s Disease Rating Scale (UPDRS), modified Hoehn and Yahr Staging Scale (HY), and UPDRS sub scores III and II with a plant-based dietary intervention.92
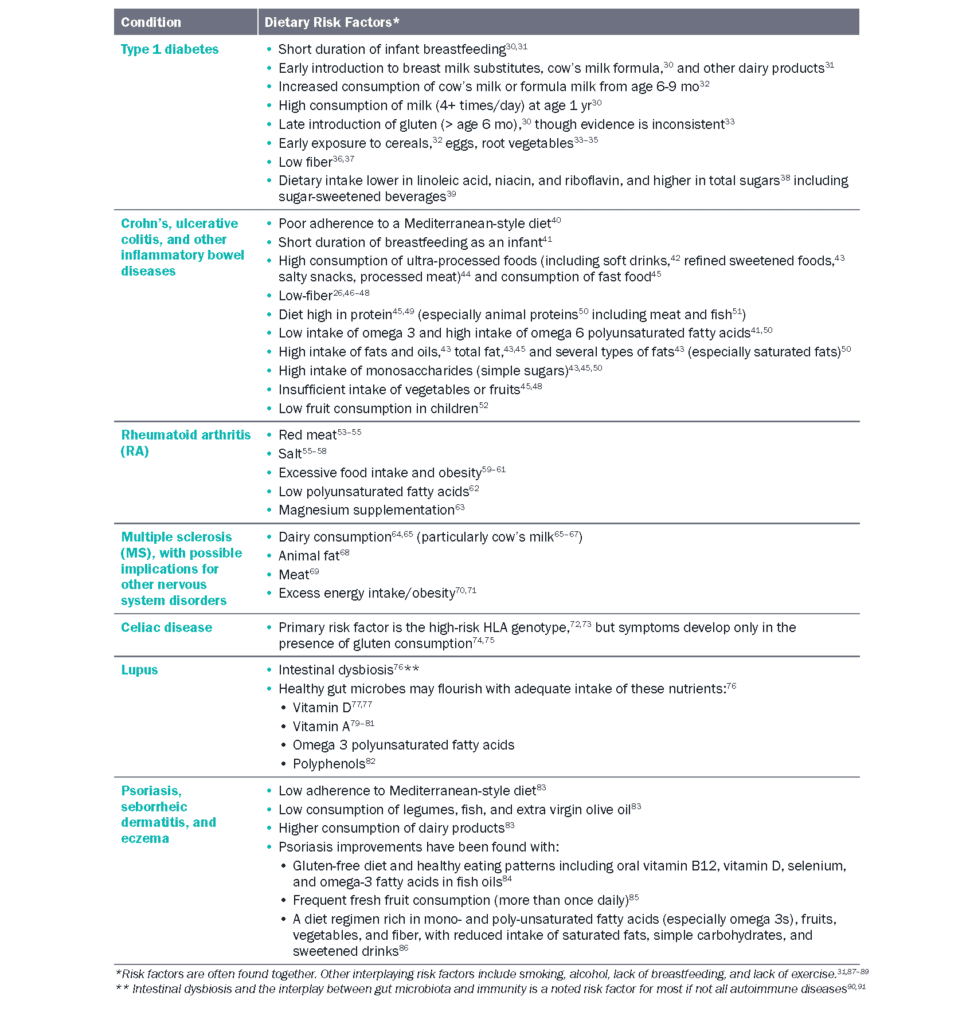
Table 1 Dietary factors related to autoimmune conditions
Benefits of Plant-Based Nutrition for Autoimmune Disease
Ample evidence suggests that plant-based diets are protective against autoimmune disease and have the potential to produce improvements in symptoms. Plant-derived antioxidants have an anti-inflammatory effect by controlling free radicals leading to oxidative stress and pro-inflammatory cytokines involved in the pathogenesis of autoimmune diseases.11, 94
Further, omega-3 fatty acids, particularly EPA and DHA found in algae and algal oil, decrease C-reactive protein levels and other inflammatory mediators and have elicited an anti-inflammatory effect in lupus patients.95, 96 DHA and EPA have also demonstrated protection against free radicals and cardiovascular alteration by reducing specific antibodies (anti-dsDNA) and interleukins (IL-1α, IL-1β, IL-2) as well as TNF-α.96, 97 Studies show their role in regulating proteinuria and blood pressure, as well.96, 97 Other sources of omega-3 fatty acids include fish, flaxseed, olives, and olive oil.98 Much research indicates that consumption of a Mediterranean-style diet that focuses on fiber-rich plant foods has been associated with reduced risk of rheumatoid arthritis (RA)99 and Crohn’s or inflammatory bowel diseases,100 though results for RA are mixed. One recent large prospective cohort did not find clear associations between iron or meat and RA,101 though several other studies have.54, 55, 99
A recent population study (N=280) found that adherence to a plant-based Mediterranean diet was associated with disease activity improvements in lupus patients, with greater dietary adherence exerting a more beneficial effect.102
Low-fat, plant-based diets have been found to lessen symptoms of multiple sclerosis and reduce or eliminate the need for medications.103–105 In contrast, diets that include animal foods have been shown to impair the management of chronic inflammatory autoimmune diseases.53, 54, 106–108
Obesity and metabolic dysfunction predispose individuals to inflammation and, therefore, autoimmune diseases.70, 71, 109 Diets higher in whole or minimally processed plant-source foods can be instrumental in maintaining a healthy weight or achieving a healthy weight if overweight or obese. Large population studies have shown that following or transitioning to a healthy plant-based diet from diets containing more animal foods is associated with less weight gain over time.110, 111
Another study showed lower baseline BMIs in vegetarians of any kind (i.e., vegan, lacto-vegetarian, lacto-ovo-vegetarian) compared with non-vegetarians. In a longitudinal follow-up, risk of obesity was 7% lower for each additional year that an entirely plant-based vegan diet was followed compared to a non-vegetarian diet.112
Dietary interventions using whole plant-based foods, particularly foods rich in prebiotics (i.e., cereal grains, legumes, fruits, and vegetables),113–115 possibly accompanied by customized medically supervised fasting,116–118 may help control and limit symptoms for certain autoimmune conditions and lessen or eliminate the need for pharmaceuticals.119–124 This dietary approach may offer a safe and effective intervention125 for this cluster of diseases that have no curative treatment in traditional medicine.
Mechanisms
There are many mechanisms by which plant foods improve autoimmune conditions. Several nutrients found in them have demonstrated a protective and anti-inflammatory effect for autoimmune disorders, including polyunsaturated fats and antioxidants, while harmful effects have been found with foods such as red meat and salt.55 A few of the foremost mechanisms linking diet to autoimmune diseases are highlighted below.
Inflammation and Oxidative Stress
Apart from normal physiological inflammation, prolonged inflammatory responses can lead to significant tissue and organ damage and are associated with many chronic diseases, especially autoimmune diseases.142 Abnormal immune response by T cells and other immune cells as well as epigenetic mechanisms have been implicated in the development and progression of autoimmune diseases,10, 142 and diet is known to be associated with inflammatory regulation in both healthy populations and those with autoimmune diseases.143
In particular, both observational and interventional studies have shown anti-inflammatory effects with the Mediterranean dietary pattern when compared to typical North American or Northern European dietary patterns.143 Similarly, the “Western diet” (characterized as high-fat and cholesterol, high protein, high sugar, and with excess salt intake, and frequent consumption of processed and “fast foods”) has been shown to be a possible promoter of autoimmune diseases. This dietary pattern leads to excessive accumulation of white adipose tissue, subsequently producing systemic inflammation through the release of pro inflammatory mediators such as TNF-α, IL-6, leptin, resistin, and C-reactive protein, and by impacting T cell response.10
A number of clinical trials have demonstrated that a low-fat vegan diet contributes to improved symptoms of rheumatoid arthritis (RA), sometimes when coupled with fasting. A randomized controlled trial (N=66) using a gluten-free, vegan diet reduced inflammation and improved the colonic microbiome, particularly lactobacilli, producing potentially atheroprotective and anti-inflammatory changes, including decreased LDA and ox LDL levels, as well as raised anti-PC IgM and IgA levels.136, 144, 145
Being in a chronic state of inflammation increases risk of cellular damage in the body through the overproduction of reactive oxygen species. This process ultimately leads to oxidative stress and damage to biomolecules (e.g., proteins, DNA);146 therefore, in addition to inflammation, oxidative stress is characteristic of many AI diseases, including lupus. The anti-inflammatory activity of omega-3 fatty acids can inhibit pro-inflammatory cytokines and C-reactive proteins. Thus, whole fruits and vegetables with intact oils can improve oxidative stress, while evidence suggests that omega 6 fatty acids in oils from processed foods are pro-inflammatory.147–151 Furthermore, many plant-based foods, such as kale and berries, are rich sources of antioxidants which are known to combat oxidative stress.152
Gut Microbiome
The role of intestinal microbiota in modulating inflammation has only recently emerged.153–156 Dietary imbalances may induce dysbiosis and introduce proinflammatory cytokines that are associated with autoimmune diseases.157–159 As evidence of this dysbiosis, several studies have shown vastly different microbial compositions in the gastrointestinal tracts of individuals with and without inflammatory bowel disease. Those with this disease have shown restriction of biodiversity and imbalanced bacterial composition compared to healthy individuals. With this change in composition also comes a distinct change in microbial functions, including fecal tryptic activity, oxidative response, or lipid and glycan metabolism pathways.160
Similarly, patients with systemic lupus erythematosus have shown restricted diversity of gut microbiota, and this is associated with increased gut permeability (“leaky” gut) and impaired gut barrier leading to less protection against pathogens in this population.161
Interactions between bacterial communities and the host can also result in epigenetic modifications that have been associated with insulin secretion and emerging conditions leading to type 1 diabetes.137 The gut may also play a key role in rheumatoid arthritis, where intestinal barrier permeability can allow endotoxins to enter the bloodstream.162 This may explain elevated antibodies and pro-inflammatory T cells in RA patients.163–165
Diet is a key factor in shaping the composition of intestinal microbiota and establishing microbiota homeostasis. Subsequently, diet plays a role in stabilizing the integrity of the gut mucosal barrier, which helps modulate gut immunity.137 Specifically, diets containing sufficient quantities of non-digestible carbohydrates, such as dietary fibers and resistant starches, which are present only in plant foods, enable gut microbes to produce short-chain fatty acids (SCFAs). These SCFAs confer beneficial effects such as reducing mucosal inflammation in the GI tract, strengthening the epithelial defense barrier to avoid pathogenic infections, and preventing insulin resistance and diabetes.137
Fiber
Increasing fiber-filled plant food improves glycemic control through normalizing beta cell function in the pancreas and allowing the body to overcome insulin resistance.166 This is especially crucial for individuals with Type 1 diabetes and those with AI diseases accompanied by obesity where glycemic control may be compromised.
Evidence supports that nutrient-dense diets high in fiber can improve glycemic load control and disease outcomes in these populations, especially when paired with exercise.167, 168, 169 For instance, studies comparing animal protein (that has no fiber) and plant protein (which contains fiber) suggest that a diet rich in plant protein may favorably affect glycemic control.170–175 Potential mechanisms explaining this benefit include the following: high intake of fiber and magnesium, elimination of oxidation produced by heme iron intake, increased intake of antioxidants (e.g., carotenoids) and other nutrients, weight loss, and the favorable amino acid profiles of plant proteins.176–178
Dietary Fats
Diets high in fat, especially those high in long-chain saturated fatty acids, have pro-inflammatory effects in many organs throughout the body.179 Studies have shown that when some or all of these long-chain fatty acids (found in animal-sourced foods) are replaced with medium-chain saturated fatty acids (found in coconut or palm oils) in the diet, incidence of spontaneous colitis can decrease, and there is some protection against chemically-induced gut inflammation due to the attenuation of pro-inflammatory cytokines and immune cell oxidative stress.179
Diets higher in unsaturated fatty acids, especially polyunsaturated fatty acids (PUFAs), along with fiber from whole plant food have been associated with decreased risk for MS as well as RA.66, 180 Among PUFAs, omega 3 fatty acids, found in plant and marine oils, have been correlated with decreased production of pro-inflammatory cytokines through decreased alkaline phosphatase and bile duct injury.179 Intake of omega 3 fatty acids at a ratio of 1 part to 3 parts omega 6 fatty acids has shown the most benefit, and studies have suggested that even partial replacement of omega 6 with omega 3 (ratio of 10) or medium-chain triglycerides can improve experimental colitis.179
Dietary patterns with higher omega 3 to omega 6 ratio, including Mediterranean-style, vegetarian, and vegan diets, have demonstrated improvements in some RA disease activity such as pain score, morning stiffness, physical function and vitality, and measures of inflammation (e.g., CRP).181
Other studies have shown that a dietary pattern low in calories and protein but high in fiber, PUFAs, vitamins, minerals, and polyphenols (e.g., whole food, plant-based diet) has the potential to modulate the inflammation and immune functions of systemic lupus erythematosus.121 In this dietary pattern, the PUFAs specifically can reduce level of inflammatory mediators, C-reactive protein, lymphocyte proliferation, macrophage-mediated and cytotoxic T-cell-mediated cytotoxicity, synthesis of proinflammatory cytokines, and chemotaxis from monocytes and neutrophils.121 Despite the growing evidence in support of diets higher in PUFAs to reduce inflammation, several randomized controlled trials have shown no effect from omega 3, omega 6 or overall PUFAs on inflammatory bowel diseases (including Crohn’s Disease and ulcerative colitis) or their associated long-term inflammatory status.182
Polyphenols and Flavonoids
Polyphenols are bioactive molecules found in plants (especially fruits, vegetables, legumes, cereal grains, olives, cocoa, tea, coffee, and wine), and flavonoids are a class of polyphenols that are especially abundant in fruits and vegetables. These compounds protect against chronic degenerative ailments in humans and have numerous health-promoting benefits, including anti inflammatory and antioxidant properties.183
For autoimmune diseases like type 1 diabetes, RA, and MS, polyphenols have a potential role in prevention and treatment by regulating signaling pathways, suppressing inflammation, and limiting demyelination.123 Polyphenols can modulate immune effects by inhibiting autoimmune T cell proliferation and downregulating pro-inflammatory cytokines (interleukin-6 (IL-6), IL-1, interferon-γ (IFN-γ)). Beyond suppressing inflammation, polyphenols can elevate antioxidant enzyme gene expression and secretion of anti-inflammatory mediators.
In inflammatory bowel diseases, polyphenols can be effective in management strategies due to their ability to modulate the expression of pattern recognition receptors and inflammatory responses in the intestinal epithelial and immune cells.
Furthermore, polyphenols can influence the gut microbiota as a probiotic, supporting the maintenance of intestinal homeostasis and reducing inflammation.123 In patients with MS, studies assessing individual flavonoid compounds have shown positive therapeutic effects alone and when combined with anti-MS therapeutic agents. Here, flavonoid compounds had anti-inflammatory effects on peripheral blood mononuclear cells (PBMCs) by reducing PBMC proliferation, reducing production of TNF-α and IL-1β from PBMCs, and inhibiting NFkB which reduced MMP-9.184
Plant-Based Diets: Summary of Key Studies
Multiple studies have examined the association between various versions of plant-based dietary interventions and improvement of autoimmune conditions, including rheumatoid arthritis (RA) (7 studies), multiple sclerosis (MS) (2 studies), and hypothyroidism (1 study). Key data are presented in Appendix Table 1.
Types of interventions included in these studies are the anti-inflammatory diet (AID), vegan diet, gluten-free vegan diet, living-foods diet, fasting followed by vegan diet, and prudent diet limiting saturated fat with slow reintroduction of low-fat animal foods. The follow-up duration for most of these studies ranged from one month to one year, with one study following participants up to 34 years.214 In patients with RA, a gluten-free vegan diet prompted reductions in various immunoglobulins and improvement in American College of Rheumatology 20% (ACR20) score.136
In a systematic review of studies on patients with RA, an intervention of fasting followed by gluten-free vegan diet resulted in statistically significant decreases in erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), swollen and tender joint count (SJC and TJC), morning stiffness, patients’ evaluation of pain, and an improvement in patients’ global evaluation.181 At one year of follow-up in another study of RA patients, there was no difference between vegetarian and control diets in ESR, SJC, TJC, but intervention participants reported significant decreases in subjective measures including pain, morning stiffness, and patients’ global evaluation compared to controls.215 In addition, studies that classified patients as “responders” tended to find more responders, or people with improvement in disease metrics, in plant-based interventions than controls.136, 181, 215, 216
Plant-based interventions demonstrated reductions in tender and swollen joint counts (TJ and SJ), improvement in global assessments of disease progress, duration of morning stiffness, grip strength, and scores on health assessment questionnaires (HAQ).128, 136, 138, 215–217
The body of work on the impact of plant-based dietary interventions in patients with MS is less clear. One plant-based dietary intervention yielded no significant improvement in number or volume of brain lesions at 12 months of follow-up.218 Another long-term intervention stud by Swank et al. found that among those diagnosed with MS who had low disability scores at baseline, 95% in the intervention group remained only mildly disabled at a follow-up of 30 years. The intervention group was assigned to a prudent dietary pattern, with the primary goal of minimizing saturated fat intake rather than limiting all animal food. Therefore, the findings in this study may only be generalizable to mostly plant-based diets with the inclusion of low saturated fat animal products.214
Finally, a 2013 study using data from the Adventist Health Study-2 (AHS-2) cohort (n = 65,981), described a lower incidence and prevalence of Graves’ disease, one form of hyperthyroidism, in people following vegan diets, compared to omnivorous, lacto-ovo-vegetarian, semi-vegetarian, and pesco-vegetarian diets, even after controlling for BMI and demographic variables. The researchers speculated that the inflammatory properties of animal products could explain the lower risk in vegans.219 However, these findings may be subject to reverse causation due to lack of a temporal component in data. In addition, none of the associations found between diet and hypothyroidism were statistically significant.219
For medical treatment, a plant-based dietary intervention may be a valuable tool for physicians to suggest to patients dealing with RA, MS, and PD symptoms, though more high-quality research must be done to solidify the effects of diet on symptoms and progression of autoimmune diseases. Most statistically significant findings are from subjective assessments from patients, making assessments vulnerable to placebo effects or response bias.
Common Questions and Concerns
Is there a link between diet and autoimmune disease?
It seems that the autoimmune response occurs from a cascade of events leading to inflammation, and that diet may be a trigger for susceptible individuals.99
Accumulating evidence shows that dietary factors are implicated in risk for autoimmune diseases,99 and a Western-style diet might increase risk.10, 53 Diets high in whole plant foods (fruits, vegetables, whole grains, seeds, beans, and nuts) and low in refined carbohydrate (sugar and flour), and saturated and trans fats (meats and dairy) appear to be protective against systemic inflammation and help to control the inflammatory response.97, 99
There does not appear to be harm in moving towards an unrefined, WFPB diet for those experiencing autoimmune diseases, whereas there appears to be a link between animal and refined foods and the inflammatory response.53, 94
Attention may be needed to avoid dietary triggers or allergic reactions to some foods or nutrients, such as gluten, for those with celiac disease. Celiac disease seems to be the exception to autoimmune diseases and diet in that there is a clear causal connection to gluten.185
Note also that those suffering from one autoimmune disorder, particularly celiac disease, are at higher risk for developing others.186 This could be due to damage done to the mucosal barrier of the intestinal lining that protects against an autoimmune reaction to pathogens entering the blood.75, 186, 187
Will a plant-based diet reduce inflammation from the autoimmune reaction?
A plant-based diet of whole grains, legumes, fruits, and vegetables that is low in or void of red meat shows potential to reduce the risk of the autoimmune response, probably due to its anti-inflammatory properties. It may be that the dietary fiber in plant foods helps to stimulate the growth of certain gut microbiota, particularly but not exclusively Bifidobacterium and Lactobacillus, which hold anti-inflammatory properties and work to improve symptoms, including inflammation and to limit joint pain.188–190
Can’t non-digestible (insoluble) fiber make IBD symptoms worse?
Though dietary fiber intake supports gut health and can control inflammation, it may be hard to determine if dysbiosis is the cause or consequence of IBD. For those with flare-ups or active IBD, Crohn’s, or ulcerative colitis, undigested fiber may worsen symptoms for some with a sensitivity to it. Further, incomplete fermentation due to the dysbiotic state may result in a buildup of pro-inflammatory metabolites, including succinate,191 that may irritate an already sensitive bowel. It may be helpful to limit intake to only the fibers with clear evidence of anti-inflammatory effects.
Some evidence indicates that limiting or avoiding insoluble fiber (brown rice and whole grains) and concentrating on soluble fibers might be most beneficial for IBD patients.192 Soluble fiber is more readily fermentable by gut microbes into the short-chain-fatty-acid butyrate. Fruits are the highest source of soluble fiber, and some research suggests long-term intake of fiber from fruits reduces risk of developing Crohn’s disease but not ulcerative colitis.193
Higher amounts of soluble fiber from whole fruits, vegetables, beans and oats may help some individuals, though certain foods may be triggers for others.194 Fruits with skin and seeds, raw green vegetables, cruciferous vegetables (broccoli, cabbage and cauliflower), whole nuts, and grains may be triggers for some. Eliminating all dairy and limiting fat intake may help.127 It may be difficult to zero in on triggers, particularly with diets that include animal protein and sugar. Working with a knowledgeable lifestyle medicine-trained dietitian or healthcare professional may be helpful.
Can a plant-based diet help patients with AI diseases lose excess weight to address AI symptoms?
Excess adipose tissue propagates inflammation and can exacerbate certain symptoms associated with AI diseases and common comorbidities, such as hypertension and metabolic syndrome, and can reduce a patient’s response to some medical treatments.117, 195, 196 Therefore, maintaining a healthy weight and reducing obesity and overweight in AI patients can be an effective treatment to reduce, alleviate, or lower the risk of developing some AI symptoms.197, 198
Plant-based diets can be effective for preventing weight gain,111, 199–201 maintaining a healthy weight,199 and achieving healthy weight loss.111, 199–202 This is largely due to the high fiber and water content of plant-based diets—especially those centered around whole, minimally processed foods—which allows most people to eat until satiated without overconsuming calories.203, 204 Furthermore, the higher nutrient-density of plant-based foods can improve nutrient status and lead to improved symptoms for patients with various AI diseases.
Does a plant-based diet provide enough protein for patients at risk of muscle-wasting due to their AI disease?
Some AI diseases, such as MS and inflammatory bowel diseases, are characterized by loss of muscle mass which can lead to lower functionality and quality of life. In these patients, a higher intake of dietary protein in conjunction with daily exercise and regular resistance training or high-intensity training may be necessary to sustain a healthy muscle mass.205–207
A well-executed plant-based diet can provide plenty of protein for all patients, including those with higher protein needs. Legumes, in particular, provide high amounts of protein as well as dietary fiber, iron, zinc, folate, and potassium, and they are low in saturated fat, which makes them a healthy alternative to animal-based protein sources such as meat and dairy.208
A daily diet that includes beans, whole grains, nuts, and seeds can provide adequate protein for most patients,209 but patients with higher protein needs may benefit from working with a knowledgeable dietitian to plan a health-promoting diet with adequate protein.
Do plant-based diets have any adverse interactions with AI treatments (since some drugs can interact with nutrients like B12 and folate)?
Several possible drug-nutrient interactions have been identified for various AI disease treatments.210, 211 Folic acid absorption can be impaired in psoriasis or rheumatoid arthritis patients receiving methotrexate. Supplementation is common for these patients.210, 211
Plant-based diets are typically high in folate since it is present in vegetables, fruits, nuts, beans, and grains. Excellent sources of dietary folate include spinach (and other leafy green vegetables), black-eyed peas, asparagus, brussels sprouts, broccoli, peas, and kidney beans.212 Psoriatic or RA patients taking ciclosporin should be advised to avoid the consumption of grapefruit juice, as this juice increases the bioavailability of ciclosporin by more than 60%.210, 211
Any patients taking systemic retinoids should be advised to avoid food sources rich in vitamin A, particularly liver, to reduce risk of hypervitaminosis A. Retinoids can also induce hyperlipidemia, so dietary patterns that are low in saturated fats and high in omega-3 fatty acids are recommended to significantly lower serum triglycerides.210
Plant-based diets are naturally lower in saturated fatty acids than diets including meat and dairy products and can include adequate omega-3 fatty acids from sources such as chia seeds, Brussels sprouts, algal oil, hemp seeds, walnuts, and flaxseeds.213 For other possible drug-nutrient interactions related to treatments for AI diseases, patients should be advised to consult with their pharmacist.
Download the full 181 page Benefits of Plant-Based Nutrition White Paper for access to all references, key studies and more.
Promising Results—Case Reports of Success with Dietary Treatment
For many AI diseases outlined here, research on associations with diet and/or nutrition is still in the early stages of exploration and needs further development through more robust studies to establish any strong conclusions. Despite this, current evidence provides support for the risk reduction and health benefits of plant-based diets in populations with various AI diseases primarily due to the strong links between diet and inflammation, immunity, and obesity. While several interventional and cohort studies have been outlined above, numerous case studies offering more dramatic examples of improvements in symptoms using diet and lifestyle interventions are also worth noting and are highlighted below.
Myasthenia gravis (MG)
In a case study reported by Yiaslas et al., a 65-year-old male veteran with multi-morbidities reversed his severe atherosclerotic heart disease, obesity, and the autoimmune disorder, Myasthenia gravis (MG), which is a neuromuscular dysfunction causing weakness in the skeletal muscles.220 After fifteen weeks on the Heart Disease Reversal Program (HDRP), which centers on a WFPB diet, this patient eliminated his angina, lowered his total cholesterol by 23% (-24 mg/dL), and lowered LDL cholesterol by 42% (-16 mg/dL).
After nine months on a WFPB diet, his musculoskeletal pain completely resolved, and he lost 20% of his body weight (50 pounds), along with improving type 2 diabetes and glycemic control metrics more than expected if he were following the American Diabetes Association diet guidelines.172 The patient reported that after two previous heart attacks and a drug-induced coma to control his MG flare-ups after surgery, he could manage and live with his disease. Nine years later, however, he decided to participate in an Esselstyn-style heart disease reversal program (HDRP) at his Veterans Hospital at Mather Field, which he says “saved [his] life.” The HDRP consists of 3 components: adoption of the WFPB diet, physical activity promotion, and stress management training.
Type 1 Diabetes
Type 1 diabetes (T1D) is an inflammatory autoimmune disease that leads to permanent degeneration, including retinopathy, nephropathy, neuropathy, foot complications, high blood pressure, and a lifetime dependence on insulin. T1D is considered irreversible, and life expectancy for individuals with T1D is estimated to be 8–13 years less than healthy non-diabetic peers.221 Despite this, compelling evidence shows how nutrient-dense diets high in fiber allow for glycemic load control and can improve disease outcomes, especially if accompanied by exercise.167, 168, 169
Fuhrman and Ferreri reported on three T1D cases who began following a nutrient-dense, plant-rich (NDPR) diet focused on vegetables, legumes, nuts and seeds, and lower-sugar fruits at different stages of T1D disease progression.169 The case reports document how insulin therapy can be lowered, delayed, or avoided altogether in T1D patients, with the potential to improve glycemic control, as well as cardiovascular risk factors. Results suggest that the earlier a WFPB diet intervention is initiated, and the younger the patient, the higher the potential to reverse the course of T1D and slow or prevent further destruction of beta cells.
In support of this, a patient who began a NDPR diet upon diagnosis at age 3 required no insulin therapy up to at least three years after diagnosis. This patient also showed a steady decline in autoantibody levels during follow-up. Another child who began a NDPR diet several months after diagnosis required only a low dose of insulin and showed a favorable HbA1c with more consistent blood glucose readings. After one month of initiating nutritional therapy, a 45-year-old male patient was able to reduce insulin requirements thirteen years after initiating insulin therapy, from approximately 70–100 units/day to 16 units of Lantus/day plus 4 units of Humalog/meal. His C-reactive protein level reduced from 4.5 to 0.2 mg/l. This patient continued to exercise regularly and weighed 149.6 lb. (67.9 kg) with approximately 10–11% body fat at the time of reporting. He mostly avoids meat, dairy, white flour products, white rice, added sugars, processed and fried foods, indicating high success in controlling his disease even without dietary perfection. These cases concur with other studies demonstrating benefits to eating this way for better control of T1D, including a boost in insulin sensitivity, more predictable blood glucose levels, reduced risk of diabetic neuropathy, kidney protection, and more energy.166, 168, 222
Lupus
Lupus is an autoimmune dysfunction causing Inflammation that can affect many different body systems, including joints, skin, kidneys, blood cells, brain, heart, and lungs. Lupus is difficult to diagnose, but the most common form is systemic lupus erythematosus (SLE). Up to 60% of SLE patients develop nephritis, a complication that causes significant morbidity and mortality.223
Goldner (2019) reports two case studies of patients who reversed symptoms of SLE, including nephritis, into remission after adopting a customized WFPB eating regimen high in raw leafy and cruciferous vegetables and rich in omega-3 polyunsaturated fatty acids, antioxidants, and fiber.224 To increase patient compliance with this diet, Goldner suggests blending specific whole plant foods into smoothies to enable patients to ingest enough nutrients from them.
In the case report, a 24-year-old female patient achieved increased kidney function (measured by the glomerular filtration rate, eGFR) from 14 to 27 ml/min in 6 weeks on her regimen, with energy and joint pain levels also significantly improved. Dialysis was no longer needed, nor was her scheduled renal transplant. Five months after beginning her regimen, the patient was considered in full remission and tapered off all medications.
The second case was a 41-year-old male with SLE who first tried a Paleo dietary pattern to control his symptoms and consumed large amounts of meat 3–4 times daily. On this diet, his symptoms rapidly worsened, developing into nephritis. He had edema in bilateral lower extremities and alopecia, and his eGFR was 61 ml/min, which put him in stage II chronic kidney disease. He first adopted a WFPB diet on his own and felt better, but he optimized recovery with Goldner’s regimen, which consisted of 454 grams of leafy and cruciferous vegetables, fruits, chia or flaxseed, and 3.8 liters of water per day through green smoothies.
Both cases displayed rapid deterioration upon deviating from the raw, WFPB intervention diet, indicating there may be even greater benefits for disease management and reversal using raw nutrients in this illness due to the availability of certain nutrients, enzymes, and changes in food structure. High consumption of meat appeared to bring on additional loss of kidney function in Goldner’s second case, which may indicate that higher protein intake, or meat intake in particular, is not optimal for SLE-related nephritis. Nephrologists who advise patients to increase animal protein intake should reconsider this advice and could alternatively recommend a highly anti-inflammatory diet that is also high in antioxidants and fiber.
Rheumatoid Arthritis
The inflammatory autoimmune condition, rheumatoid arthritis (RA), attacks the joints by causing inflammation and damage to the tissue. It can also affect other tissues throughout the body and cause problems in organs such as the lungs, heart, and eyes.225 Specific causes of RA are unknown, and the primary treatment includes medications that can be costly and have side effects. However, some evidence suggests that dietary intake can impact the severity of RA symptoms. For instance, saturated fat may be linked to worsened RA symptoms, while monounsaturated fats are associated with improved outcomes.94, 136
Other research suggests that dietary fiber can improve the diversity of gut microbes which may significantly impact RA progression as described above. McDougall et al. (2002) reported using a very low-fat vegan diet over four weeks for 24 patients with RA and found significant improvements in RA symptoms (joint pain, stiffness, swelling, and function; p < 0.001).136 On this diet, patients decreased intake of fat (69%), protein (24%), and energy (22%), and significantly increased intake of carbohydrates (55%). All measures of RA symptomatology decreased significantly (p < 0.05) except for duration of morning stiffness (p > 0.05). Weight also decreased significantly (p < 0.001). At 4 weeks, non-significant decreases were found for C-reactive protein (-16%, p > 0.05) and RA factor (-10%, p > 0.05), while erythrocyte sedimentation rate was unchanged (p > 0.05).163
Treatment using a WFPB diet to mitigate inflammation and weight alone makes it a valuable tool to consider for RA and inflammatory diseases in general.
Conclusion
The studies reviewed here indicate the value and effectiveness of using WFPB dietary intervention to control and, in some cases, potentially reverse autoimmune disorders. Compared to other tools available to practitioners, dietary interventions are inexpensive, have few side effects, and come with co-benefits such as weight maintenance, cardiovascular improvements, improved brain function, decreased fatigue, and the establishment of lifestyle improvements. These diseases merit further rigorous research, and more randomized controlled studies comparing the effects of plant-based diets to other dietary interventions in populations with autoimmune diseases are needed. In conclusion, several studies to date have reported lower risk of autoimmune diseases and improvement of symptoms with a WFPB diet.
Download the full 181 page Benefits of Plant-Based Nutrition White Paper for access to all references, key studies and more.
CONTINUE READING IN THIS SERIES
- The Benefits of Plant-Based Nutrition
- The Benefits of Plant-Based Nutrition: Diet Quality
- The Benefits of Plant-Based Nutrition: Obesity & Weight Management
- The Benefits of Plant-Based Nutrition: Treatment and Prevention of Type 2 Diabetes
- The Benefits of Plant-Based Nutrition: Treatment and Prevention of Cardiovascular Disease
- The Benefits of Plant-Based Nutrition: Treatment and Prevention of Chronic Kidney Disease
- The Benefits of Plant-Based Nutrition: for Enteral Nutrition
- The Benefits of Plant-Based Nutrition: Treatment and Prevention of Reproductive Cancers
- The Benefits of Plant-Based Nutrition: Treatment and Prevention of Autoimmune Disease
- The Benefits of Plant-Based Nutrition: Longevity and Quality of Life
Acknowledgement
This review was made possible in part due to a generous donation from Kate Farms. For more information on Kate Farms please visit their website here. www.katefarms.com








