The benefits of plant-based nutrition: Treatment and prevention of type 2 diabetes
Plant-predominant diets are associated with decreased risk of developing type 2 diabetes across all age and sex categories. Even small amounts of meat consumption have been shown to increase the risk of developing diabetes.
March 31, 2023

Abstract
The prevalence of diabetes is rising in the United States and worldwide, posing a major public health concern. There is an urgent need to curb this rapidly rising incidence. Epidemiological studies have found a lower prevalence of type 2 diabetes (T2D) among plant-based (vegetarian/vegan) populations as compared to nonvegetarians.
Nonvegetarian diets are rich in calorie-dense foods, such as processed foods, refined grains, and animal derived products, which influence metabolic abnormalities leading to insulin resistance, obesity, and diabetes.
Dietary interventions using whole food, plant-based (WFPB) diets have been highly effective in the prevention and treatment of diabetes, offering a safe and effective way to achieve serum glycemic control and insulin homeostasis. In addition, WFPB diets promote weight loss, which is a primary surrogate of insulin resistance (IR) in most individuals. Healthy plant-based diets not only improve IR but also improve the common modifiable cardiovascular risk factors, including serum lipids, serum glucose concentration, and systolic and diastolic blood pressure.
The current treatment for T2D centers around medications which slow the progression of the disease without achieving remission or cure, whereas dietary lifestyle changes of adopting a WFPB diet can not only prevent progression of diabetes but also achieve remission. Healthy plant foods—whole grains, vegetables, legumes, fruits, nuts, and seeds— are within reach of many individuals living with T2D. Diabetes remission should be the primary goal in the management of T2D. The emerging outcome data in many studies suggest that plant-based diets may be a practical solution to prevent and treat chronic diseases, including diabetes.
Table of contents
Key points for practitioners
Diabetes in context
Benefits of plant-based diets for type 2 diabetes
Mechanisms by which plant-based diets act to address T2D
Common questions and concerns
Summary of key studies
Promising results – Examples of disease remission
Conclusion
Key points for practitioners
- WFPB diets can be used not only for prevention but also for the treatment of T2D.
- The attributes of WFPB diets that are particularly helpful for diabetes treatment include the lower fat content, high fiber content, and high water content,19 leading to overall lower energy density.
- Appropriate dosing19 of intensive, therapeutic lifestyle change is essential in using diet for diabetes treatment. Transitioning to a low-protein, low-fat, plant-based diet has delivered better results than simply reducing meat and adding more vegetables.19
- Due to the potentially rapid effects of plant-based dietary treatment for T2D, caution and close glucose monitoring with patients on medications are advisable, as there is the potential for sudden drops in blood glucose and blood pressure levels.19
- Heme iron is absorbed at a higher rate from animal foods, and its pro-oxidative effect can promote insulin resistance.20, 21
- Using a plant-based diet to restore insulin sensitivity and treat T2D has the potential to improve other chronic conditions as it reduces inflammation, the unifying mechanism in metabolic, obesity, and cardiovascular disorders.7, 10, 12, 14, 15, 19, 22
- If patients express interest in trying a WFPB diet of some kind, it is helpful to share ACLM patient-facing resources and tools for practical guidance on plant-based eating.
Learn to send diabetes into remission with our flagship CME/CE course Remission of Type 2 Diabetes and Reversal of Insulin Resistance with Lifestyle Medicine
Diabetes in context
Diabetes mellitus is a group of metabolic disorders in which either the pancreas does not produce enough insulin to regulate blood glucose, or the body cannot use insulin effectively.1 This results in hyperglycemia. Chronic hyperglycemia leads to damage, dysfunction, and failure of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels.2, 3 Clinically, diabetes is diagnosed when hemoglobin A1c (HbA1c) is 6.5% (48 mmol/mol) or higher,1 and HbA1c 5.7–6.4% (39–46 mmol/mol) is considered prediabetes.4
Diabetes mellitus is a global health problem with a worldwide prevalence of 8.8% and is associated with multiple comorbidities and high health care costs.5 The Centers for Disease Control report that 10.5% of the US population have diabetes and 34.5% have prediabetes.6 Many of those with diabetes are undiagnosed, with estimates indicating that 26.9 million people are diagnosed and 7.3 million are un- or underdiagnosed.6
Of the diagnosed diabetes reported in the US, 90% are type 2 diabetes (T2D).7 Patients with T2D typically show abnormalities in their lipid metabolism, such as higher levels of fasting and postprandial triglycerides, higher levels of small, dense low-density lipoprotein (LDL) particles, and lower levels of high-density lipoprotein (HDL) cholesterol.5 This state can further worsen betacell dysfunction and IR, increasing the incidence for unfavorable macrovascular endpoints (e.g., cardiovascular disease (CVD), myocardial infarction, and CVD mortality) and microvascular complications.5 It is important to improve the management of T2D in order to achieve both glycemic and lipid goals.5 T2D is now the leading cause of blindness in developed countries as well as the leading cause of kidney disease through its microvascular complications.8 Blood pressure (BP) levels in T2D patients are, on average, higher which is the primary cause of cardiovascular diseases in these patients.8
Of the different types of diabetes categorized, the most common is type 2, in which the body produces insufficient insulin or cannot use insulin effectively. The focus of this report is T2D, as this condition is lifestyle-driven, and its causes are largely modifiable, particularly with diet.
Benefits of plant-based diets for type 2 diabetes
Plant-predominant diets are associated with decreased risk of developing T2D across all age and sex categories.9 Even small amounts of meat consumption have been shown to increase the risk of developing diabetes.10–13 One longitudinal study examining adult Seventh-Day Adventists without diabetes at baseline (N=8,401) found that after 17 years of follow-up, those who consumed meat just once per week had a 29% higher risk of developing diabetes than those who refrained, and this risk increased to 38% if the meat was processed. Lifelong adherence to a vegetarian diet in this population was associated with a 74% reduced risk of developing diabetes compared to a diet that included weekly meat consumption.13–15 As shown in Figure 1, T2D prevalence in this population among meat-eaters remained approximately twice that of individuals avoiding meat.13, 15
Consumption of processed meat five or more times per week is associated with increased risk of T2D. In the Nurses’ Health Study II, there was a 91% increased risk of diabetes among participants who reported eating meat (n=91,246).16 Consuming red meat 5 or more times per week compared to <1 time per week, increased risk of T2D by 59%. These studies suggest that while a Western dietary pattern is associated with overall diabetes risk, meat consumption is independently associated with increased risk for diabetes.13 Diet as a modifiable lifestyle factor plays an important role in treating diabetes.17
The American Diabetes Association (ADA) recommends an individualized medical nutrition therapy for glycemic and lipid management, and no single dietary approach for patients with T2D has been recommended, though a common theme emerges for successful dietary intervention that includes a high fiber, plant-based diet.8 Research consistently shows that meat consumption is associated with increased diabetes risk,13 while vegetarian diets rich in fiber and low in saturated fat are the most beneficial for the prevention and treatment of diabetes. Plant-based diets are inversely associated with risk of developing diabetes, independent of the positive association of meat consumption with diabetes development.13, 17 Although more long-term intervention trials are required, mounting evidence supports the view that vegan, vegetarian, and plant-based dietary patterns should be clinically used in both medical and public health contexts to achieve better glycemic control in individuals with T2D,18 as well as treatment for patients with a goal of remission.19[vc_column_text el_]
Mechanisms by which plant-based diets act to address T2D
Excess weight and insulin resistance have long been identified as the underlying causes of T2D.23 Mechanisms by which plant-based diets act to address these causes to reduce diabetes risk include maintaining healthy weight and lipid mass, both overall and within pancreatic and hepatic cells. These benefits lead to protection of beta-cell function, a decrease in inflammation, improved insulin sensitivity, and avoidance of IR.9, 19
Beta-cell function (and mass)
Caloric intake exceeding energy needs causes glucotoxicity (hyperglycemia) and lipotoxicity (elevated free fatty acid levels), which disrupt glucose and insulin metabolic relationships. Pancreatic lipotoxicity inhibits beta-cell insulin production, reducing available insulin to maintain glucose homeostasis. Impairment of insulin secretion worsens over time, paralleled by progressive decline in both pancreatic B-cell function and mass, ultimately leading to persistent hyperglycemia.24 Caloric restriction has demonstrated the rapid correction of glucotoxicity and lipotoxicity, leading to the removal of intracellularly accumulated triglycerides (and their precursors) and restoration of beta-cell insulin production.19, 25
Heme iron
The highly absorbable heme iron in meat acts as a prooxidant that leads to the production of reactive oxygen species (ROS), known to damage the insulin-producing pancreatic cells.26 Even moderately elevated iron stores are associated with increased risk for T2D.21, 26, 27 Iron overload increases risk for IR, T2D, and cardiovascular disease due to increased fatty acid oxidation.27, 28 Increased oxidative stress in the pancreatic islet cells has been observed in mouse models.28 Conversely, reductions in stored iron, through either dietary changes or phlebotomy, have been found to increase insulin sensitivity by 40%.21, 26, 29 The lower iron stores in vegetarians compared to meat-eaters appear to improve insulin sensitivity and enhance glucose disposal.21
Inflammation
Inflammation is suggested to be the unifying pathogenic mediator for IR, excess weight, T2D, and cardiovascular diseases.30 In the human diet, saturated fatty acids (SFA) are derived from animal sources,31 while trans-fatty acids (TFAs) originate in the meat and milk of ruminant animals31 resulting from bacterial biohydrogenation of unsaturated fatty acids in the rumen. Partial hydrogenation of unsaturated fatty acids in vegetable oils during the industrial production of certain foods produces TFAs, as well.32 Most TFA has similar physical properties to SFA. Excess intake of SFA or TFA can promote lipotoxicity in several target organs by direct effects represented by inflammatory pathways by complex activation of toll-like receptor pathways (TLR-4 and TLR-2) releasing inflammatory cytokines, responsible for chronic systemic inflammation.32
The high-fat, low-fiber Western diet promotes the overgrowth of gram-negative pathogens, with consequent increased intestinal translocation of bacterial lipopolysaccharides resulting in endotoxemia from the toxic byproducts.33 This interacts with specific cellular receptors of the host’s immune system (TLR-4/CD-14), culminating in an inflammatory cascade that precedes the development of insulin resistance, obesity, diabetes, and cardiovascular disease.33 At the cellular level, the adaptive inflammatory response is exerted through TLRs signaling the lipopolysaccharides from gram-negative bacteria, CpG DNA, and flagellin.34 A 2014 Harvard study reported that as total red meat consumption increased among women from the Nurses’ Health Study, so did biomarkers of inflammation.35
Branched-chain amino acids
Animal-derived protein is rich in branched-chain amino acids (BCAAs).36, 37 Meat and dairy (whey protein and casein) are rich sources of leucine.31 Leucine, isoleucine, and valine are essential BCAAs important for regulation of growth, protein biosynthesis, and metabolism, but also appear to contribute to obesity-related IR.38 Elevation of BCAAs in human obesity was first reported in 1969.38 Recent advances in the metabolic role of BCAA have been recognized as a “metabolic signature” predicting insulin resistance in human subjects.38, 39 The Western-style diet provides conditions for excessive stimulation of mammalian target of rapamycin complex 1 (mTORC 1), a critical nutrient-sensitive enzyme that promotes growth and cell proliferation in response to glucose, energy, growth factors, and amino acids. This can lead to insulin resistance, diabetes, and obesity.38 In contrast, plant-derived polyphenols and flavonoids are identified as natural inhibitors of mTORC1 and exert antidiabetic and anti-obesity effects.40
Energy density
Plant-based diets are rich in fiber with significant water content from vegetables and fruits and low in fat. As a result, they have low energy density. In many individuals, this allows for ad libitum eating resulting in satiety with less total energy consumption.41, 42 Conversely, animal-derived products high in fat and without fiber make them high in energy density leading to excess consumption of total energy.43 Dietary interventions with plant-based foods, void of meat and other animal-derived products, usually result in low total energy consumption conferring beneficial effects in weight loss and glycemic control.13, 44 Insulin Resistance and Intracellular Lipids Insulin resistance and excess weight appear to have a bi-directional relationship,45 usually resulting from excess caloric intake. Excess calories lead to the deposition of ectopic fat in the liver, pancreas, and muscle. In the pancreas, lipotoxicity inhibits beta-cell function by reducing insulin production. In most organs, intracellular fat interferes with glucose uptake by affecting the glut-4 transporter, even before weight gain.46 This suggests that excess energy can rapidly promote insulin resistance due to increased oxidative stress in a matter of days, even before excess energy is stored to cause weight gain.47
Studies using magnetic resonance imaging have demonstrated lipid deposition in muscle and liver cells which leads to insulin resistance.48 Intervention trials have shown plant based diets can improve insulin resistance and reverse diabetes.19
Besides general adiposity, visceral fat contributes to insulin resistance, possibly from increased proinflammatory cytokines originating in visceral fat cells and adipose tissue-resident macrophages.49 Intracellular fat accumulation within muscle and liver cells appears to aggravate insulin resistance which, in turn, contributes to type 2 diabetes.50–52 Possible mechanisms suggest insulin resistance is aggravated by the specific amino acids and fat that are particularly abundant in meats and that saturated fatty acids in meat products may increase the insulin response which, in turn, increases the respiratory quotient and reduces fat oxidation.53
Whole food, plant-based diets
Glycemic control can be achieved (in many cases without medications) by avoiding excess calorie consumption, either by reducing the volume of food or by choosing low energy-density foods. WFPB diets, naturally low in fat and high in fiber, satisfy these measures, having the key attributes that improve glycemic control, insulin resistance, and beta-cell function.19
Meat products are generally higher in fat content than grains, legumes, vegetables, and fruits.54 Not only can higher dietary fat increase excess energy content and contribute to the accumulation of intracellular lipids, but high fat foods also appear to downregulate the genes responsible for mitochondrial oxidative phosphorylation in muscle tissue.55 Among those who avoid animal products, intramyocellular lipid concentrations are significantly lower, compared with age- and weight-matched omnivores (−9.7, 95% CI −16.2 to −3.3, P = 0.01).56 T2D remission has only been demonstrated with consumption of a very low-energy diet,57 and the diet that offers the greatest reduction in fat content is a WFPB diet.58
Therapeutic dosing
As with pharmaceutical therapeutics, appropriate dosing19 is imperative to achieve an intended outcome. Similarly, achieving remission in T2D requires a decrease in basal insulin secretion in patients and increased glucose sensitivity, just as aspirin for pain relief requires 325-650 mg once or twice a day.10, 19, 25, 41, 59, 60 Practitioners have found the greatest success in achieving diabetes remission using an intensive dose of a WFPB dietary treatment.19 However, caution must be exercised regarding the potential of sudden drops in blood glucose and blood pressure, especially if the patient is on medications for these conditions, which may require urgent adjustment.9
Common questions and concerns
But I thought you had to avoid carbs to control blood sugar?
Refined carbohydrates such as white flour and sugar are fast absorbed from the gastrointestinal tract, entering the circulation quickly, and this burdens the pancreas to produce insulin in excess amounts. On the other hand, unrefined carbohydrates in whole foods with their high fiber content slow the absorption rate of glucose with an attended slow rise in blood glucose level, thereby decreasing the demand on the pancreas for insulin release. This, in turn, improves insulin sensitivity. High complex carbohydrate-based diets have been shown to decrease blood glucose levels and HbA1c.9, 61
Isn’t a Keto diet good for diabetes?
A ketogenic diet may help maintain a low level of blood sugar and help with weight loss in the short term, but it does not address IR.19 The excess fat intake leads to the deposition of triacylglycerol, the storage form of fat, in the liver and pancreas, causing lipotoxicity, the root cause of diabetes.62 As a result, meat consumption over time is highly predictive of weight gain, ultimately leading to insulin resistance.9, 14 In addition, toxic compounds from the breakdown of meat by the gut microbiome, namely trimethylamine and its conversion to trimethylamine N-oxide (TMAO) and advanced glycation end products (AGE) have been implicated in the pathogenesis of T2D,63 as have nitrosamines in processed meat and the heme iron in meat.64
What about low-carbohydrate diets in general for remission?
Overall safety concerns exist regarding the cardiometabolic effects of low-carbohydrate diets,65 and long-term observational studies in humans have not been conducted. Higher red and processed meat intake have been consistently associated with increased risk of T2D as well as other chronic diseases, including cardiovascular disease, which should arouse concern.66
Plant-based diets: Summary of key studies
Plant-based diets offer encouraging results in improving T2D risk profiles, glycemic control, weight loss, and blood lipids. We extracted 12 of the highest-quality studies from our review to analyze the effect of plant-based diets on markers of T2D risk, symptom management, and glycemic control (see Appendix Table 1). Of the data extracted, three studies were randomized controlled trials (RCTs),25, 67, 68 one was an observational cohort study,11 five were meta-analyses,69–73 and three were systematic reviews.18, 74, 75 All studies examined one form of low-fat, plant-based diet, vegan diet, or vegetarian diet, compared to a control or comparison diet. Outcomes of interest were changes in weight, waist circumference (WC), body composition, HbA1c, glucose sensitivity, insulin resistance, and changes in blood lipids.
Of the randomized controlled trials, one study found that a long-term, low-fat vegan diet intervention was as effective in reducing weight and HbA1c as the ADA guidelines. The vegan diet had greater decreases in both HbA1c and weight compared to the ADA diet, though these group-by-time interactions were not significant.67 Another study examining a brown-rice-based vegan diet intervention found that the intervention group had greater decreases in HbA1c and waist circumference than the control.68 Another RCT found that plant-based dietary intervention was associated with decreases in BMI, visceral fat volume, fat mass, insulin resistance, glucose sensitivity, basal insulin secretion, C-reactive protein, and improved homeostatic model assessment for insulin resistance (HOMA-IR) scores.25 An observational cohort study within the Adventist Health Study (AHS) cohort found that vegan and lacto-ovo-vegetarian diets were associated with decreased incidence of T2D.11
Overall conclusions in systematic reviews were that plant-based dietary patterns improved markers of glycemic control, such as reductions in HbA1c. In addition, a plant-based dietary intervention resulted in weight loss and reduction in the need for T2D medications. Successful diets in the management of T2D all centered on plant foods: Mediterranean, vegan, vegetarian, DASH diet, and Ma-Pi 4 (macrobiotic) diets.18, 74, 75 One meta-analysis found that a vegetarian diet was associated with lower odds of T2D compared to control diets. Subgroup analysis found that men on vegetarian diets had greater reductions in odds of T2D compared to men in control groups, whereas there was no effect of vegetarian diets for women and Southeast Asian populations, suggesting heterogeneity among populations.70 This discrepancy may be explained by the regional difference in understanding and practice of a vegetarian diet. For example, Indian vegans may eat greater amounts of butter or ghee (clarified butter), which differs from the vegan dietary practice of Western countries.76, 77
Another meta-analysis examining observational cohort studies found a significant reduction in risk of T2D with vegan and vegetarian dietary patterns. This study also found a dose-response relationship between adherence to a plant-based dietary pattern and T2D.72 Another meta-analysis of RCTs found that a vegetarian diet was associated with decreased HbA1c, fasting glucose levels, LDL-C, weight, and BMI.73 Though there was some heterogeneity across systematic review and meta-analysis outcomes, the majority of studies examining plant-based dietary effects on T2D show reductions in HbA1c, weight, blood lipids and cholesterol, and improvements in glycemic control and insulin sensitivity. Plant-based dietary interventions should be considered as a low-cost treatment for risk reduction and symptoms of T2D.
Promising results—Examples of type 2 diabetes disease remission
Appropriate dosing and high patient adherence in the context of lifestyle modification are essential to achieving T2D remission. Researchers clarify that “remission has not been reported with inadequately dosed lifestyle changes, such as eating more salad, or simply reducing meat consumption.” However, “remission is achievable for a majority of short duration (<8 years) T2D patients, and many with longer duration, with sufficiently intense LM interventions.”19,62,78
Rapid remissions
Lim et al’s 2011 case-control study (n=11) exemplifies appropriate therapeutic dosing of diet and lifestyle intervention showing immediate remission of T2D in 1-4 weeks. (49.5 ± 2.5 years, BMI 33.6 ± 1.2 kg/m2, nine male and two female) were studied before and after 1, 4, and 8 weeks of a 2.5 MJ (600 kcal)/day diet. Basal hepatic glucose output, hepatic and peripheral insulin sensitivity and beta cell function were measured. After one week of restricted energy intake, fasting plasma glucose normalized in the diabetic group (from 9.2 ± 0.4 to 5.9 ± 0.4 mmol/l; p = 0.003). Insulin suppression of hepatic glucose output improved from 43 ± 4% to 74 ± 5% (p = 0.003 vs. baseline: controls 68 ± 5%). Hepatic triacylglycerol content fell from 12.8 ± 2.4% in the diabetic group to 2.9 ± 0.2% by week 8 (p = 0.003). The first-phase insulin response increased during the study period (0.19 ± 0.02 to 0.46 ± 0.07 nmol min−1 m−2; p < 0.001) and approached control values (0.62 ± 0.15 nmol min−1 m−2; p = 0.42). Maximal insulin response became supranormal at 8 weeks (1.37 ± 0.27 vs. controls 1.15 ± 0.18 nmol min−1 m−2). Pancreatic triacylglycerol decreased from 8.0 ± 1.6% to 6.2 ± 1.1% (p = 0.03).62
Case study: Reversal from “healthy” to plant-based diet
A 44-year-old woman with grade 3 obesity, T2D, and depressed left ventricular heart function (heart failure), eliminated her diabetes by adopting a healthy plant-based diet and improved her cardiovascular health. She lost 22.7kg in 5 1/2 months, and her ventricular systolic function normalized. At the same time, she was able to discontinue all glucose-lowering medications. This case demonstrates the feasibility of dietary interventions for individuals in reversing metabolic comorbidities.
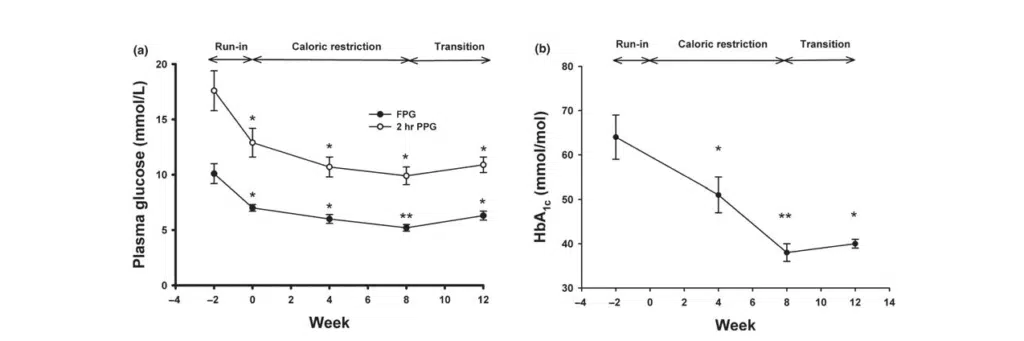
(a) Fasting plasma glucose (closed circles) and 2-hr plasma glucose after an OGTT (open circles) were measured at weeks −2, 0, 4, 8, and 12.
(b) HbA1c was determined at weeks −2, 0, 4, 8, and 12. *: p < 0.01, **: p < 0.001 compared to values at week −279
Figure 2 (a) Changes in fasting plasma glucose (FPG), 2-hr plasma glucose after an OGTT (PPG), and (b) HbA1c during the study periods.
Conclusion
Cumulative evidence demonstrates that T2D can be prevented and treated without pharmacological interventions or surgery, using a WFPB dietary regimen. Remission of T2D should be the primary clinical goal, and this is feasible for many individuals using a low-fat, WFPB diet. This treatment is shown to be well-tolerated and facilitates weight loss without feelings of hunger or deprivation.80
CONTINUE READING IN THIS SERIES
Acknowledgement
This review was made possible in part due to a generous donation from Kate Farms. For more information on Kate Farms please visit their website here. www.katefarms.com
Take the Next Step
Ready to turn knowledge into action? Gain practical tools and skills to support plant-based nutrition for your patients and community:
- Our Food as Medicine Jumpstart Guide is free, comprehensive PDF packed with tips, meal ideas, and actionable strategies to help patients get started with plant-based eating.
- Our Culinary Medicine Program is a dynamic, self-paced online program designed to help clinicians translate the science of nutrition into practical skills patients can use in the kitchen..
Empower yourself and your patients with the skills to make lifestyle change practical, achievable, and delicious.